Cis 1,4 di-tert-butyl cyclohexane††††††††††††††††††††† †††††††††† ††nader makarious††††
How did I do it?
I
started with ChemDraw where I draw cis-1,4-di-tert-butylcyclohexane using
the dash wedge tools, and then I copied the molecule and pasted into Chem3D.
On
Chem3D to rotate the molecule around different axes and directions
trying to see the chair conformation which was really hard at the beginning.
Changing
colors, atoms size, different angles and getting rid of hydrogen atoms helped me
for better understanding.
After
I saw the chair conformation I tried to push hydrogenís atoms apart as far I
could, to reduce any kind of steric interaction.
After
it looked good enough I choose the mm tool to minimize the energy and as a
result I got a twisted boat formation which showed me the minimum energy of 27.4662 .
I believe the conformation shows the minimum energy because itís apparent that all hydrogen atoms are in the farthest position away from each other.
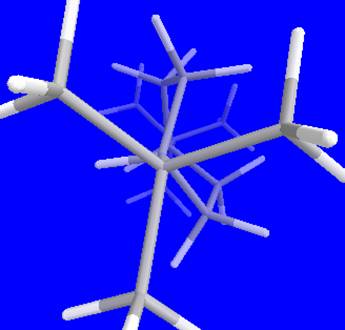
Itís also clear in this picture
all bonds are staggered (gauche) and eclipsed
conformation doesn't exist.